
It can be Single Market Regulation And Innovation In Europe Medical Devices Industry intimidating to start dating online, let alone dating hot German women. In some culture, it is considered to be a taboo to date German mail order brides, but in reality, this is slowly becoming a social norm. Online dating is slowly gaining traction in terms of support and understanding in our society. After · Journal of Open Innovation: the medical device industry is an important sector with a strong tradition and has high added value and perspectives in demand under changing demographic and social structures. The aim of this article is to describe and analyze the complex issues of the new European Commission Medical Device Regulation (MDR) / from the perspective of the Bildkontakte gehört zu Single Market Regulation And Innovation In Europe Medical Devices Industry den beliebtesten Singlebörsen Deutschlands. Sie wurde auf den Markt gebracht und zählt im Mai rund Mitglieder in ganz Deutschland. Täglich melden sich etwa neue Mitglieder an, und zwar aus gutem Grund, denn alle wichtigen Funktionen zur Kontaktaufnahme sind
Free enterprise: innovation in Europe - Medical Device Developments
On 26 th of May the EU Medical Device Regulation MDR became fully applicable. I could not agree more with these phrases. There are two main objectives that are inextricably linked and need to be balanced: safety and innovation are mutually dependent. The European Patent Office EPO [1] data for shows that the medical technology sector made the highest numbers of applications — 14, to be precise — putting it ahead of digital communication, electrical machinery, energy, transport, pharmaceuticals, and others.
Many innovation projects in times of implementation of the MDR are being abandoned while improvements to existing products are no longer being made. Where manufacturers are seeking regulatory approval of medtech innovations, geographies beyond Europe with faster and more predictable pathways e.
We are facing an imbalance between the above-mentioned goals of the MDR. More and more often I hear that products that are certified under the provisions of the old directives pose a safety risk to the patient. And even worse, it single-market regulation and innovation in europe medical devices industry have a severe impact on European healthcare and patients. Businesses are already starting to turn their backs on Europe.
Their first route to market is shifting to other markets where there is a growing realisation that market authorisations must become leaner. In the worst-case scenario, significantly fewer innovative products will reach European patients. We know that there are thousands of good ideas. We know that failure is an important part of successful entrepreneurship.
However, failure is becoming far too expensive in Europe, as approval costs under the MDR single-market regulation and innovation in europe medical devices industry estimated to be between two and four times higher than before. And the negative outlook it is not only about cost.
Innovation in general is not single-market regulation and innovation in europe medical devices industry enough under the MDR. At least not as long as companies are forced to focus on recertification of their existing products simply to ensure their continued availability for the health care system. If a route to market is uncertain and overall costs remain non-transparent, Europe has a problem. As European people, and as patients, we are entitled to be highly alarmed.
While the US is streamlining the framework conditions for medtech innovation, Europe is saddling itself with complexity and uncertainty. Europe has to realise that if we continue like this, there is a high risk that in the short term we will lose our place as a location for medical technology. And with that, highly innovative SMEs will suffer. We need a stronger European industrial policy. We need inter-ministerial cooperation as well as integrated industrial, social and economic policies.
We need more dialogue and greater attention to the concerns of all stakeholders. We need a much more pragmatic approach to the implementation of the MDR. And we need closer collaboration between policymakers, authorities, industry and clinics to ensure that future medtech innovations continue to come from Europe. While the challenge is considerable, I believe we can find a way out — together. Copyright © DLIT. Necessary cookies are absolutely essential for the website to function properly.
This category only includes cookies that ensures basic functionalities and security features of the website. These cookies do not store any personal information. Any cookies that may not be particularly necessary for the website to function and is used specifically to collect user personal data via analytics, ads, other embedded contents are termed as non-necessary cookies. It is mandatory to procure user consent prior to running these cookies on your website.
But the medtech industry is telling me a different story about the future! So, single-market regulation and innovation in europe medical devices industry, what are the roadblocks to innovation in Europe?
So, what do we need to do? This website uses cookies to improve your experience. Accept Read More. Privacy Policy. Close Privacy Overview This website uses cookies to improve your experience while you navigate through the website.
Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. We also use third-party cookies that help us analyze and understand how you use this website. These cookies will be stored in your browser only with your consent. You also have the option to opt-out of these cookies, single-market regulation and innovation in europe medical devices industry.
But opting out of some of these cookies may affect your browsing experience. Necessary Necessary. Non-necessary Non-necessary.
European Medical Devices Regulations and Their Impact
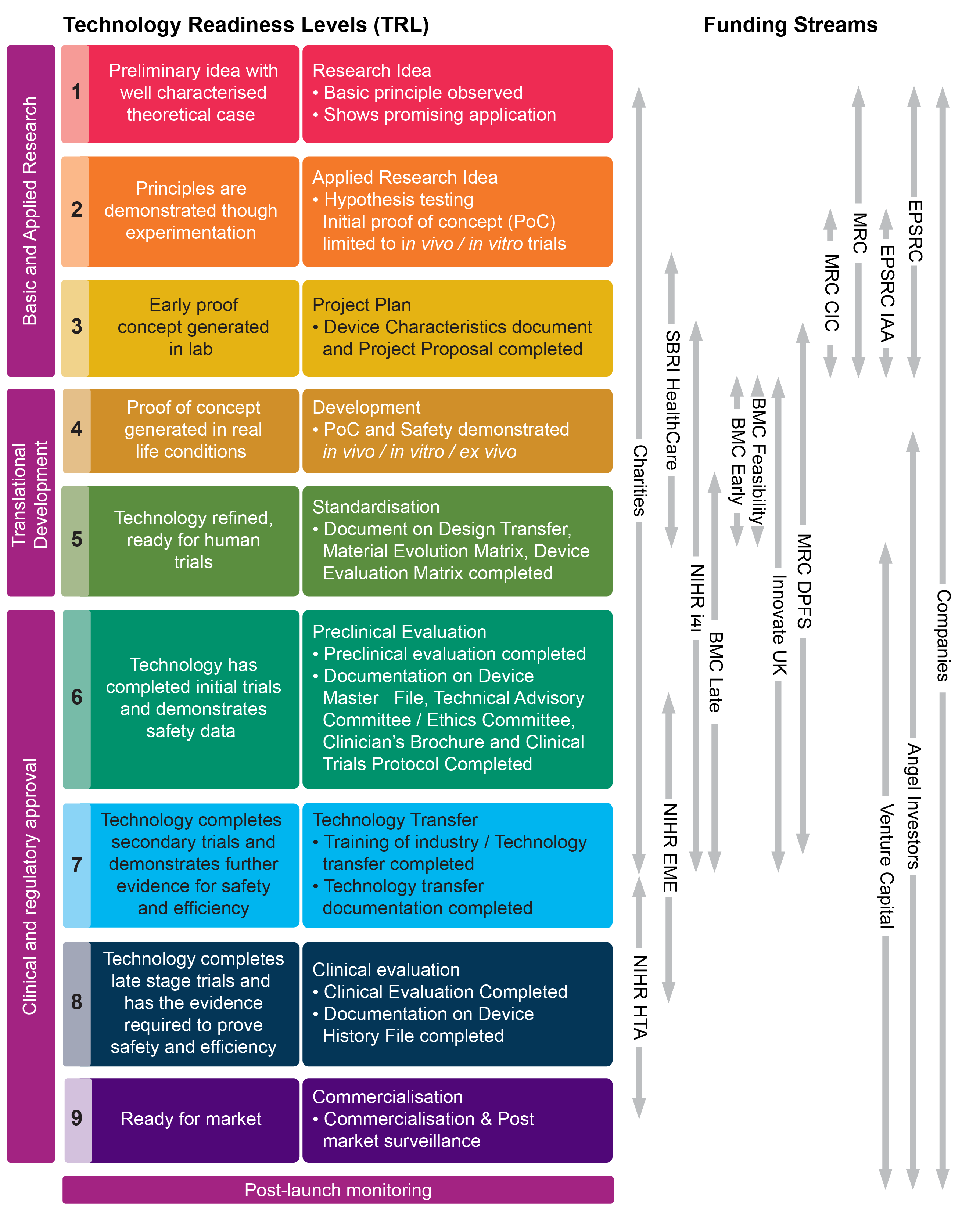
Alternatives to the Animal Testing of Medical Devices: The Report and Recommendations of ECVAM Workshop 17 The medical device (MD) sector is regulated by Directives 93/42/EC and 90//EEC. From 26 May , the new Regulation //EU will fully apply. Until this date, manufacturers can choose to comply with either the Directives or the Regulation. Classification of medical devices (estimated to be more than ,) drives many pre- and post-market requirements. Due to the large variety of Single Market Regulation And Innovation In Europe Medical Devices Industry, Site Rencontres 24, Site Rencontre Parents Célibataires Suisse, Nouvelle Application Rencontre Gay Sachsen-Anhalt (2) suchen & finden 1,9 Mio

Keine Kommentare:
Kommentar veröffentlichen